MagSi-cfDNA
Circulating cfDNA by Liquid Biopsy
from plasma, serum or urine
Home > Products > Nucleic Acid Purification > MagSi-cfDNA
A Liquid Biopsy is a non-invasive medical test that involves analyzing a sample of body fluids, typically blood, to obtain information about a person’s health status. Unlike traditional biopsies, which involve extracting tissue samples through surgical procedures, liquid biopsies provide valuable insights into various aspects of health and disease through the analysis of molecules present in the bodily fluids.
MagSi-cfDNA
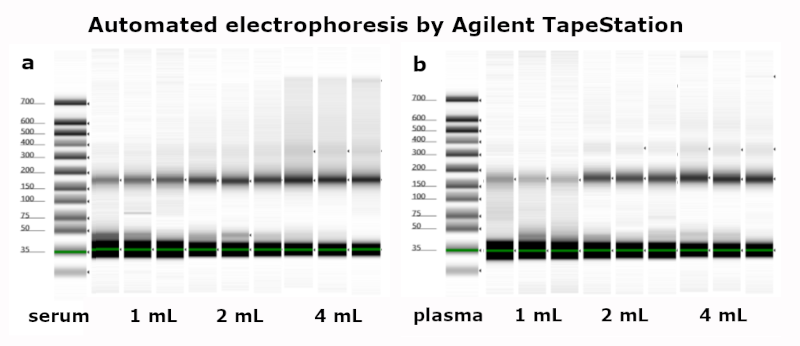
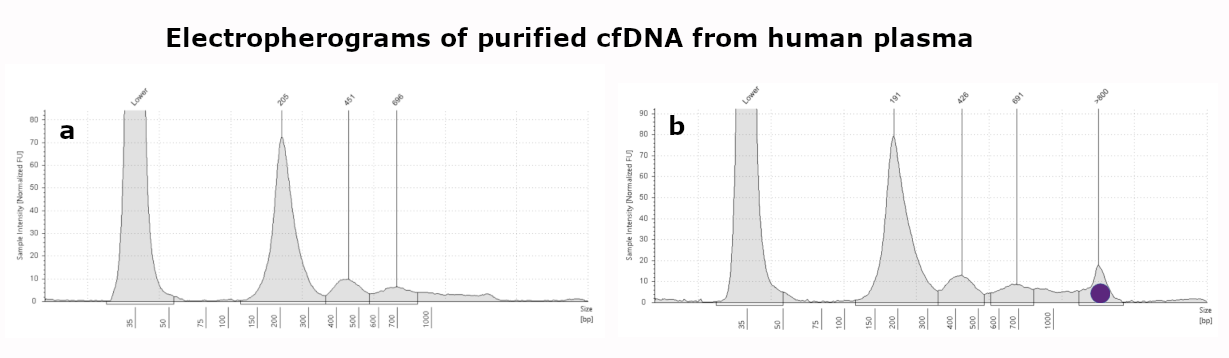
This kit is intended for Liquid Biopsy, the (automated) purification of circulating cell-free DNA from human plasma, serum or urine samples. Processing time for the preparation of 24 samples is about 60 minutes.
Safety
The kit requires no phenol/chloroform extraction or alcohol precipitation and eliminates the need for repeated centrifugation, vacuum filtration or column separation. It allows safe handling of potentially infectious samples. The obtained cfDNA can be used directly for downstream applications such as qPCR, or any kind of enzymatic reaction.
Features
- Optimized for (automated) use on PurePrep 24
- Suitable for use with fresh or frozen plasma, serum or urine samples
- Plasma can be collected with various blood collection tubes (Streck Cell-Free DNA BCT, EDTA, Citrate etc.)
- Kit provides reagents for 96 extractions of cfDNA from 2 mL sample
- Scalable for use between 1 and 4 mL sample, support protocol for 10 mL sample volume available
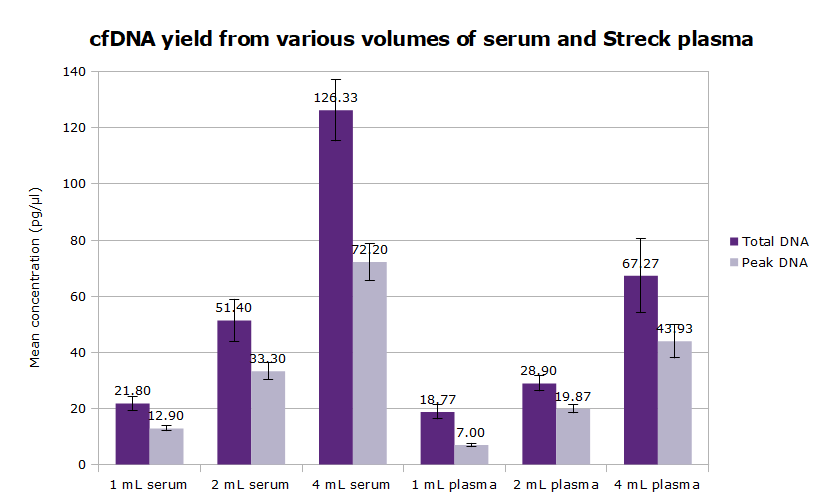
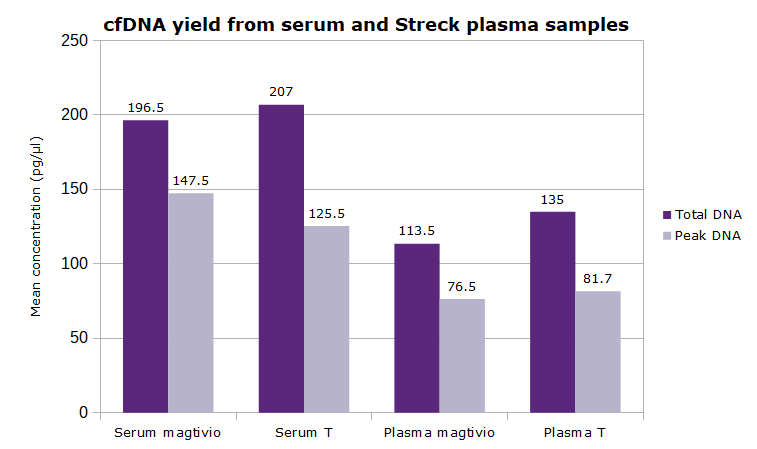
- Typical yield: 0.5 to 4 ng cfDNA per mL of human plasma (but highly variable from donor to donor)
- Following lysis at 56°C all other steps are processed at RT
- Does not require carrier RNA
- Magnetic beads are supplied in an optimized storage buffer for decreased sedimentation time
- Suitable for many enzymatic down-stream applications in particular RT-qPCR and sequencing
- Processing time for 24 samples: ~60 min
- Kit comes complete (no additional alcohols required)
Now also from urine samples, scalable between 1 and 10 mL
|
ART.NO.
|
DESCRIPTION
|
AMOUNT
|
|---|---|---|
|
MDKT00220096
|
MagSi-cfDNA
|
96 preps
|